Introduction
Herspiegel worked with their cross-functional client team to build and maintain a highly complex project plan and launch strategy for a Phase 3 asset in two different therapeutic areas. The strategy needed to take account of a rapidly changing and increasingly competitive market.
The Problem
A client recently acquired a Phase 3 asset, that needed to be launched in two separate therapeutic areas, within 6 months of each other. Their commercialization team needed to establish a process to identify and collate market insights and pressure test launch strategies for both therapeutic areas. They also needed to develop robust launch plans with various scenarios built-in and begin executing with a resource plan that involved stakeholders with varying priorities.
Our Solution
• Herspiegel compiled existing primary/secondary research to help refine the overall strategies around each launch.
• Facilitated workshops around the clinical data and key launch strategies to pressure test them among a team of internal and external experts.
• Collaboratively developed, refined, and socialized an integrated cross-functional US launch plan for launches in both therapeutic areas with all key stakeholders.
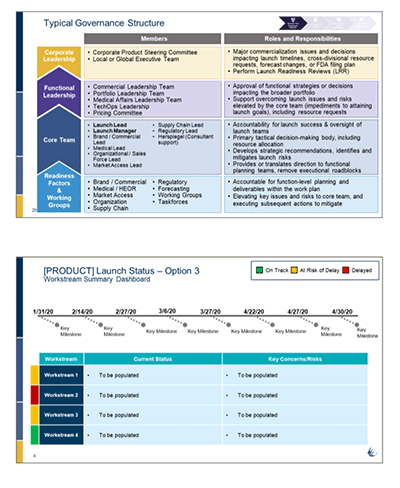
• Established a governance process for the product launches; managing project workstreams and teams to ensure rapid and seamless integration of internal and external stakeholders with timely updates to senior management.
Value Delivered
• Successful launch strategies that offered proper direction to a team with many competing priorities.
• Supplied clear and concise project plans detailing strategy, key initiatives, and tactics ensuring both alignment and coordination.
• Successful launches within both therapeutic areas with the brand exceeding first year and second-year goals by more than 25%.
Do you want to assess your launch readiness?
We have experience in more than 75 launches, spanning cell & gene therapy, oncology, rare disease, diabetes, obesity, infectious disease, CNS and trauma.


