Best Practices to De-risk Your Launch Plan
After many years of drug development and establishing a go-to-market strategy, you are finally at the tactical launch planning stage. Getting to this phase is a significant accomplishment, but this does not guarantee commercial success. In fact, 40% of products underperform analysts’ forecasts by more than 20%. At Herspiegel, we have partnered with biotech and pharma companies big and small and have successfully brought millions of dollars worth of drugs, devices, and treatments to market. In this four-part blog series, we outlined the essential components of our proven go-to-market model.
- Where to play?
- How to win
- How to execute
- Launch Plan
De-risk Your Launch Plan
This post focuses on the best practices for launch planning, developed from our experience in more than 70 pharmaceutical product launches.
Today there is a laser focus on product performance out of the gate, pharma companies need to make sure that they have the right launch team before putting together the structure and tactics in their launch plans. With all the time and resources already invested into the product, the cost of failing is high. This is why pharmaceutical and biotech companies come to us to ensure that their launch plans are exactly what they need to make their products successful.
Launch Plan
The launch plan needs to be appropriate to the organization’s situation and phase, Learn, Build, Govern, and Track. It gains detail as the company progresses through the launch stages, ensuring there is enough planning to help inform strategic decisions and spending. As commercialization nears thirty-six months, more detailed plans help ensure appropriate market shaping and insight development at the right time. The essential components of a launch plan are launch management, launch readiness assessment, and launch summit.
Launch Management
Having a plan to check against and drive timely decisions critical to launch management. When working on a drug launch, you need to have structure and process so that the 10-20 teams involved stay aligned. This usually consists of a governance structure that can help fortify alignment between the teams and keep the communication and information flowing.
This central governance team will develop the launch plan and strategy, outlining critical activities and timelines. The governance structure and team are crucial to ensuring these interdependent activities are aligned. This allows launch teams the ability to accelerate and de-risk the launch. Managing this plan and the operational support team ultimately guides decision-making during launch execution.
Launch Readiness Assessment
Before launching and after communicating the plan and process to all the stakeholders, it is time to assess launch readiness.
A Launch Readiness Assessment includes six key areas:
- Market access
- Supply chain
- Organizational
- Stakeholder
- Customer
- Marketplace
Many organizations assess their commercial readiness via a launch simulation exercise. This two-day off-site gathers diverse stakeholders, including the brand team, market access, sales leadership, medical affairs, regulatory, global, and a handful of other cross-functional teams. Groups are assigned to companies/brands already on the market in the same area/space as their product or treatment.
On Day 1: we assume the role of the established player and come into the meeting having completed a couple of hours of pre-work (read the PI, market share and volume data, payor landscape, etc.). An example of a scenario for simulation: how to anticipate and react to another brand’s launch, e.g., what would be said promotionally, what tactical levers would be pulled, and what should be done from a rebating standpoint.
On Day 2: we put our own brands’ hat back on and say, “OK, if the ‘other brand’ is going to do/say XYZ, we need to be prepared to counter with ABC.” The workshop helps to pressure test how competitive the commercial space is, how entrenched current competitors are, and the importance of existing brands to their respective portfolios.
When in Launch Mode, it’s easy for companies to focus solely on the “Days to Launch” countdown clock and not look at their blind spots. The launch simulation exercise forces companies to look at the market through an objective, competitive lens.
Launch Summit
The objective of holding a launch summit is for all the stakeholders in the cross-functional teams to share recent developments and data to align all the teams on current assumptions. This helps validate and inform adjustments to the launch plan.
Goals for the launch summit include:
- Pressure test final plan to identify interdependencies, gaps, and execution risks
- Ensure coordinated hand-offs and appropriate cross-functional phasing of activities
- Ensure cross-functional teams visibility, alignment, and readiness
- Resolve minor issues and flag major issues for discussion post-workshop
The stakeholders and teams that should participate are:
- Market Access
- Patient Services
- Regulatory
- Market Insights
- Sales
- Medical
- Marketing/Brand
- Corporate Communications
- Trade/Distribution
- Commercial Ops
- R&D
The launch summit sets the tone for the company and product team and says a lot about the organization’s culture. Some companies will throw a big party and make it fun, and others will make it all business with a 12-14 hour workday. Ultimately, it’s an opportunity for leadership to set the tone and expectations for their teams.
An example of good content for a launch summit would include the following:
- A patient testimonial (live, if possible)
- Market landscape overview from a key opinion leader (KOL)
- A discussion of unmet needs and opportunities for the launch
- Presenting differentiating attributes that will be reinforced during sales training workshops
- Methods for navigating “pillars and potholes,” which are the attributes HCPs like about the product and the Achilles heel the competition will be whispering about
- A workshop on Key Performance Indicators, or what good looks like for sales and how to hold themselves accountable
- Sales teams should talk through local challenges at the regional level
The output of the launch summit should provide a final launch plan that is aligned cross-functionally and pressure-tested for gaps and inconsistencies. The Core Team will identify the program-level risks and challenges to prioritize post-summit and pre-launch. Each Expert Team should walk away with a clear set of interdependencies, risks, and open questions. At a good launch summit, the leadership wraps up with a rallying cry and call to action. Launch meetings are inherently exciting and should be celebratory.
Summary
The drug launch landscape has become increasingly complex; many new medicines target narrow patient populations with complex distribution and delivery. Even if your product is not a specialty medicine, you are still likely to face intense price pressure in a crowded and competitive market.
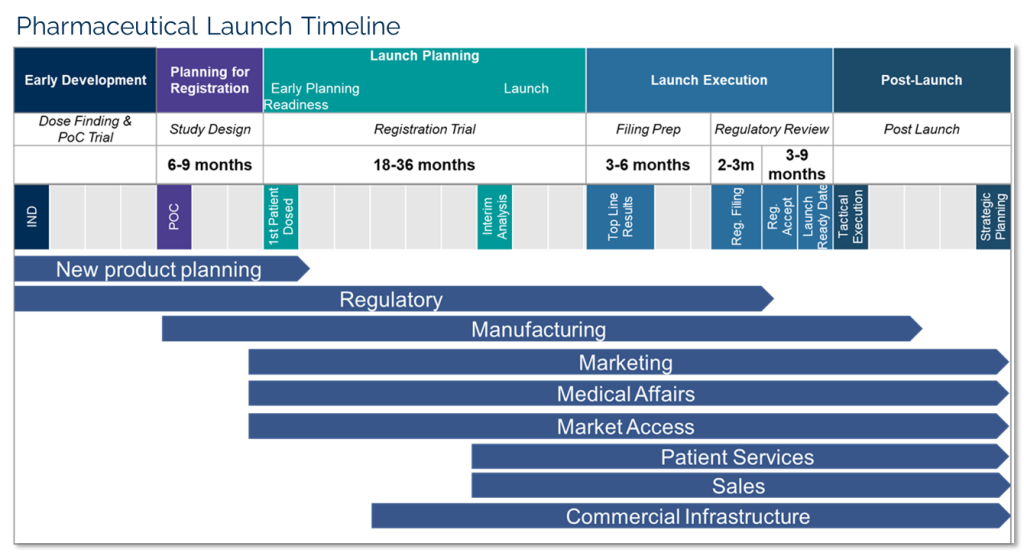
With all of this in mind, pharmaceutical and biotech companies face many challenges to ensure a successful launch, with only one chance to get it right. A new drug launch trajectory is determined in the 12-18 months before launch. An experienced team following a proven go-to-market framework can de-risk and accelerate launch.
Do you want to assess your launch readiness?
We have experience in more than 75 launches, spanning cell & gene therapy, oncology, rare disease, diabetes, obesity, infectious disease, CNS and trauma.


