How are pharma companies preparing for this contingency?
A Complete Response Letter (CRL) is issued by the FDA in response to a complete review of data in an NDA, ANDA, or BLA submission. CRLs decline approval of a drug or biologic, due to outstanding questions following data review. In the document, the FDA outlines the deficiencies detected in the submission and may offer recommendations of actions to secure approval. Following receipt of a CRL, the sponsor has one year to re-submit the required additional data based on the letter’s recommendations. On average, a company that receives a CRL experiences delay in product approval by fourteen months and takes seven months to initiate a response. Read more about trends in CRLs, how to minimize the risk and delay in our whitepaper: PREPARING YOUR LAUNCH FOR A COMPLETE RESPONSE LETTER (CRL) CONTINGENCY.
There has been a significant increase in CRLs over the past three years. As of May 2022, there have been sixteen CRLs issued to a mixture of both large and small businesses. These have had a substantial impact on the entire industry, but it is felt more strongly by small companies. Of the CRLs issued this year, there has been an almost equal split between quality and clinical concerns. It has become known that the FDA is seeking additional information in clinical trials regarding methods and devices used in efficacy endpoints.
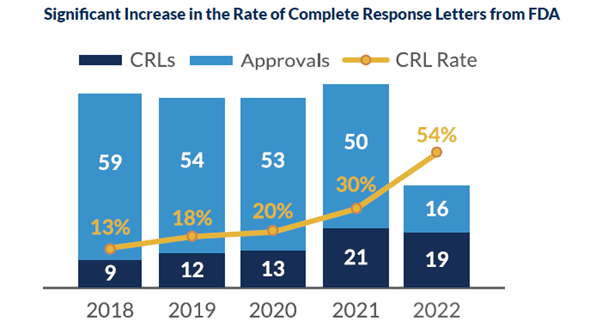
Receiving a CRL may seem dire, but there are several measures that can be taken to prevent the potential altogether. In May 2022, the FDA published draft guidance for manufacturers outlining multiple actions to avoid the possibility of a CRL.
If a CRL ends up being issued there are three main alternatives on how to proceed:
- Address issues and resubmit application to FDA for further review
- Withdraw the application
- Request a hearing on the grounds for why the CRL was given. This final route is dependent on agreement on behalf of the FDA
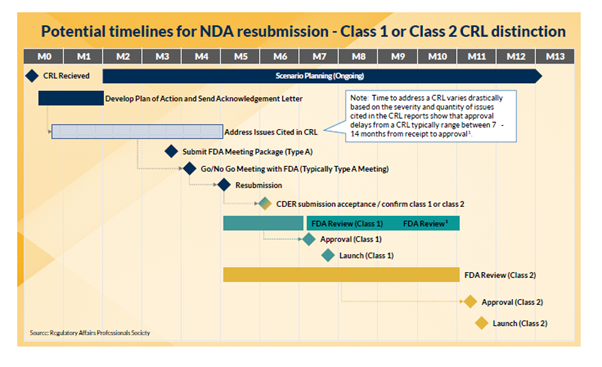
As the volume of CRLs increases (particularly among novel agent applications), Commercial teams should be prepared for this less-than-ideal possibility and incorporate it into their launch planning. Receiving a CRL does not need to be a “death sentence.” In fact, many products have gone on to blockbuster status. Herspiegel’s team works with clients to build and maintain launch plans that are robust enough to account for many possibilities, including a CRL. Scenario planning throughout, and previous experience, enable our team to help navigate the organization through what is often an uncertain time. Read more about how to minimize the risk and delay from a CRL in our whitepaper: PREPARING YOUR LAUNCH FOR A COMPLETE RESPONSE LETTER (CRL) CONTINGENCY.
Does your plan include CRL scenarios?
With reputations and revenue at stake, companies need to be prepared for a CRL to minimize the impact. An experienced launch partner can help you prepare for and respond to a potential CRL.

