What Pharma Manufacturers Need to Know about the Inflation Reduction Act
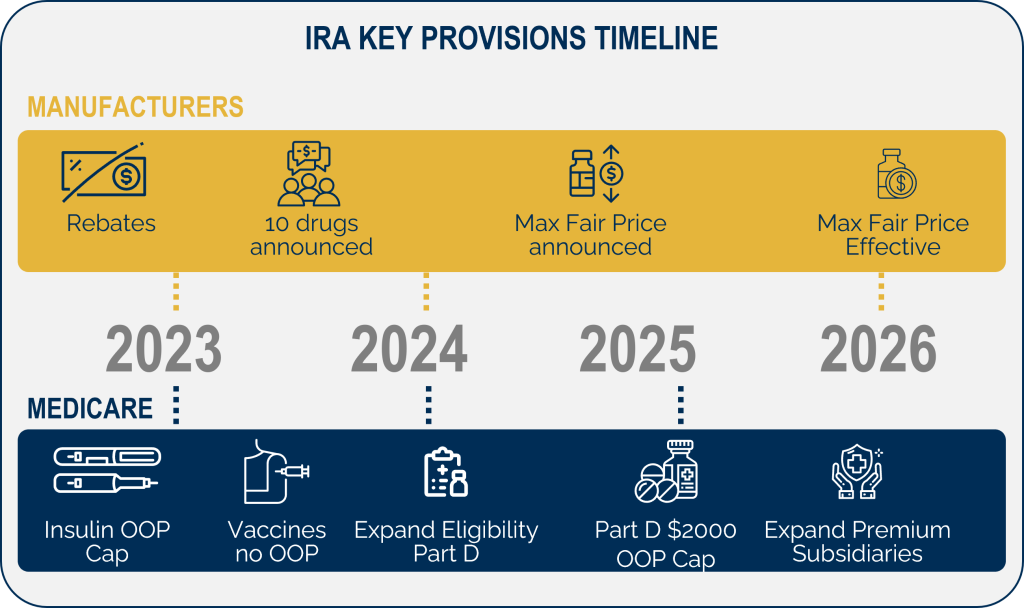
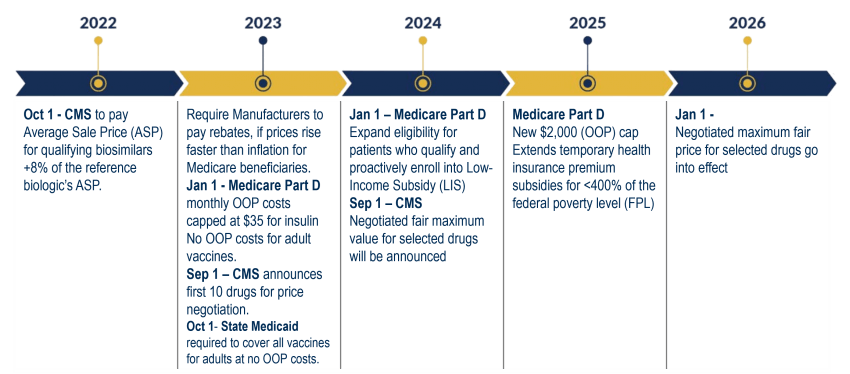
On August 16, 2022, President Biden signed the Inflation Reduction Act (IRA) into law (P.L. 117-169). The IRA is a comprehensive law that includes multiple provisions to provide financial relief for Medicare beneficiaries, improve access to care, while working to reduce overall drug expenditures by the federal government.
Herspiegel has been working with clients to help understand the impacts, stay up to date as new guidance is released by the Centers for Medicare & Medicaid Services (CMS) and prepare for the effective dates of the key provisions. The IRA will make many new improvements to the Medicare program overall, bringing innovative, life-saving treatments to patients. Many on the pharmaceutical manufacturing side are focused on preparing for the Medicare Drug Price Negotiation Program (or ‘Negotiation Program’), while that potentially has the largest impact, there are other key provisions to be aware of.
We will continue to study these new provisions and the impacts as they evolve. We are committed to sharing that information with you. Sign up below to stay up to date as we release new articles and whitepapers.
GET THE LATEST UPDATES ON IRA POLICIES AND GUIDANCE
Updates on new provisions, policies, guidance, and impacts of the IRA.
"*" indicates required fields


