Last updated: December 19th, 2025
NICE’s decision to revise its cost-effectiveness threshold marks a meaningful evolution in the UK market access landscape. The updated range is expected to support broader access to innovative medicines while offering greater clarity for pricing, reimbursement, and launch planning through 2028.
How NICE’s Cost-Effectiveness Threshold Is Being Updated
NICE has confirmed a revision to the cost-effectiveness threshold used in Technology Appraisals, increasing the range from £20,000 to £30,000 to £25,000 to £35,000 per quality-adjusted life year (QALY). The change, linked to the UK–US Economic Prosperity Deal, was presented to the NICE Board during its recent public meeting (1).
This adjustment reflects a shift in how value is assessed within the UK health technology assessment (HTA) framework.
What This Update Could Mean for Highly Specialised Technologies
It remains uncertain whether similar revisions will be applied to the Highly Specialized Technologies (HST) threshold for ultra-rare conditions. NICE has stated it will consult with the Department of Health & Social Care (DHSC) before determining whether changes to the HST framework should be pursued.
Why the Threshold Change Matters for Patient Access and Industry Planning
The increase in the NICE CE threshold represents a significant policy development with the potential to expand patient access to innovative therapies. NICE has indicated that the updated threshold may enable 3 to 5 additional treatments or indications to become available each year (2).
The impact of this change is further reinforced by broader UK pricing reforms, including the proposed three-year 15% cap on the VPAG rebate. This cap is notably lower than the 22.5% rebate in 2025 and closely aligned with the 14.5% rebate confirmed for 2026 (3).
Together, these developments represent one of the most consequential access-focused shifts in recent years and provide improved predictability for manufacturers planning UK submissions and launches through at least 2028.
What to Expect Next in the Implementation Process
On December 9th, the government launched a consultation to introduce regulatory powers allowing the DHSC to formally set the revised CE threshold. Following this process, NICE is expected to open a separate consultation detailing how the updated threshold will be applied in practice (4).
How Ongoing NICE Appraisals Will Be Managed
NICE has confirmed that current appraisals will continue under existing timelines. However, specific provisions will apply in some instances.
For appraisals with committee meetings scheduled before the revised threshold is implemented, where a product does not meet the current threshold but would be considered cost-effective under the new range, publication of the final draft guidance will be temporarily paused. Once the updated threshold is in effect, anticipated no earlier than April 2026, NICE would proceed to publication (5).
NICE has also confirmed there are no plans to retrospectively reassess previously published guidance (5).
Expected Timeline for NICE Threshold Implementation
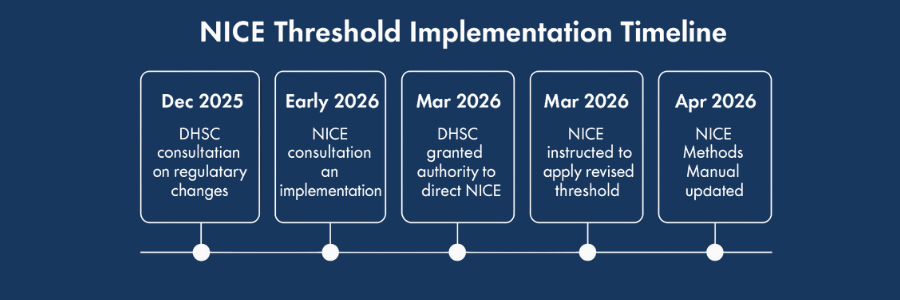
Information Source (4)
How Herspiegel Delivers Impact
As NICE’s cost-effectiveness framework evolves, manufacturers face new considerations across pricing strategy, HTA evidence development, and UK launch planning. Herspiegel experts support teams in translating policy change into actionable access strategy.
Our teams can help with:
UK HTA and NICE readiness, including value strategy aligned to revised CE thresholds
Pricing and reimbursement scenario planning under VPAG and emerging policy reforms
Evidence and economic modeling to support cost-effectiveness and value demonstration
Launch sequencing and submission strategy for specialty and rare disease portfolios
If you would like to explore how these changes may affect your pipeline or upcoming submissions, connect with our expert team for a focused discussion.
Meet with our Global Value and Access Team
Meet The Author

Gaia Geraci
Manager
Gaia has several years of experience in life sciences consulting, spanning market access and health policy. Her work has focused on conducting research, analysis, and delivering strategic recommendations to clients to drive commercial success and to help shape systems to enhance access to medicines.
Visit similar content:
FAQs on NICE
1. Why has NICE revised its cost-effectiveness threshold?
The revision reflects broader policy objectives under the UK–US Economic Prosperity Deal and aims to align better NICE’s assessment approach with innovation trends and access priorities.
2. What is the new NICE cost-effectiveness threshold?
The revised threshold for Technology Appraisals is £25,000–£35,000 per QALY, replacing the previous £20,000–£30,000 range.
3. Will the change apply to ultra-rare disease assessments?
This has not yet been confirmed. NICE is seeking guidance from DHSC on whether updates to the HST threshold will be required.
4. How will the update affect ongoing appraisals?
Appraisals will proceed as scheduled, though final guidance may be paused in cases where cost-effectiveness would only be achieved under the revised threshold.
5. When is the revised threshold expected to take effect?
Subject to consultation outcomes, implementation is anticipated in April 2026, following regulatory updates and revisions to the NICE Methods Manual.






