Since CMS announced the first ten drugs selected for price negotiation on August 29th, 2023, primary manufacturers had until October 1st, 2023, to sign agreements to participate in the Price Negotiation Program. By October 2nd, 2023, manufacturers participating must have submitted extensive amounts of their required data and information to help inform negotiations.
The public also has a chance to submit data on therapeutic alternatives or information for the selected drugs using the public portal by today, October 2nd. After submitting an email request to receive a public submission form, CMS sends an email indicating, “Once you begin to answer the questions, you CANNOT SAVE your work for that selected drug. You will need to complete all information you would like to share for each selected drug in one sitting. Once you submit a response for one selected drug, you can use the same link to return and submit responses for other selected drugs (up to 10), if you would like.”
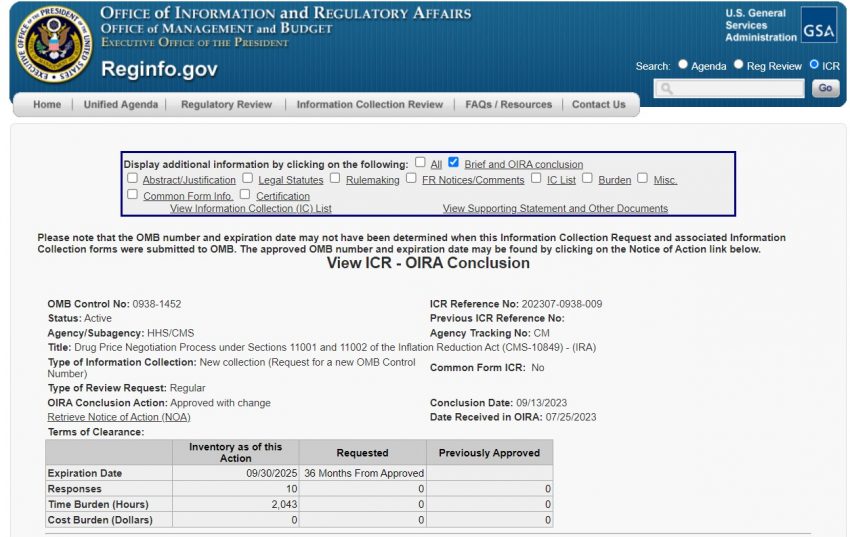
As of October 2nd, 2023, there have been ten responses submitted thus far, with all 10 appearing to have their submissions ‘approved’. CMS does indicate the ‘annual time burden’ being 2,043 hours to date. CMS indicates the ‘annual cost burden’ being $0, which may not necessarily be counted the same by the various manufacturers. However, the great news is it appears that no submissions have been rejected thus far.
CMS has posted a new Counteroffer Form, which in accordance with section 1194(b)(2)(C) of the Act, can be submitted if CMS’ written initial offer is not accepted by the primary manufacturer. The primary manufacturer can submit this form no later than 30 days after the date of receipt of the written initial offer.
Click here for the latest Medicare Drug Price Negotiation Program fact sheet from CMS.
We will continue to monitor The Office of Information & Regulatory Affairs (OIRA) website for updates, given the impending deadlines for information to be submitted. Please feel free to reach out to us if you have any additional questions regarding this update.
GET THE LATEST UPDATES ON IRA POLICIES AND GUIDANCE
Updates on new provisions, policies, guidance, and impacts of the IRA.
"*" indicates required fields

