The Centers for Medicare & Medicaid Services (CMS ) just announced the first set of drugs and biologics for price negotiations to be effective 2026. Many manufacturers have been anxiously awaiting the initial list since the passing of the Inflation Reduction Act (IRA) last year. As many know, this will allow CMS to negotiate prices directly with manufacturers in efforts to lower healthcare costs to the Medicare program and for Medicare beneficiaries.
Approximately 8,247,000 people with Part D covered used these drugs to treat a variety of conditions (cardiovascular, diabetes, autoimmune diseases, cancer), accounting for $50.5 Billion in total Part D drug costs, or 20% of Part D gross covered prescription drug costs between June 1, 2022, and May 31, 2023.
The drugs selected are single-source drugs with at least seven years since FDA approval (11 for Biologics) with the highest Part D gross covered prescription costs. Many drugs were anticipated based on Medicare Part D Drug Spending and Utilization, Calendar Years 2017 – 2021 reports; however, a few may have been a surprise.
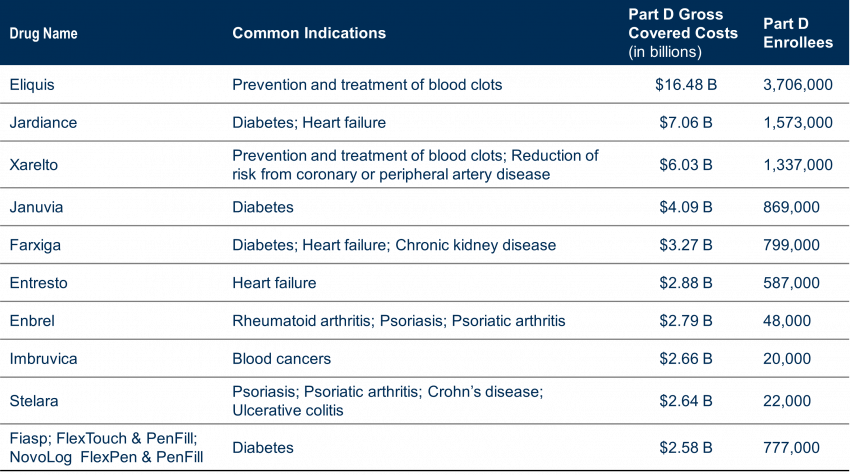
The initial list includes 6 insulin products– which supports President Biden’s ongoing efforts to not only cap insulin costs at $35 per month (started this past January 1, 2023) but to encourage manufacturers of insulin to also willingly lower their drug prices in efforts to meet that call.
Click here to see the CMS fact sheet for the Price Negotiation Program with the selected drugs.
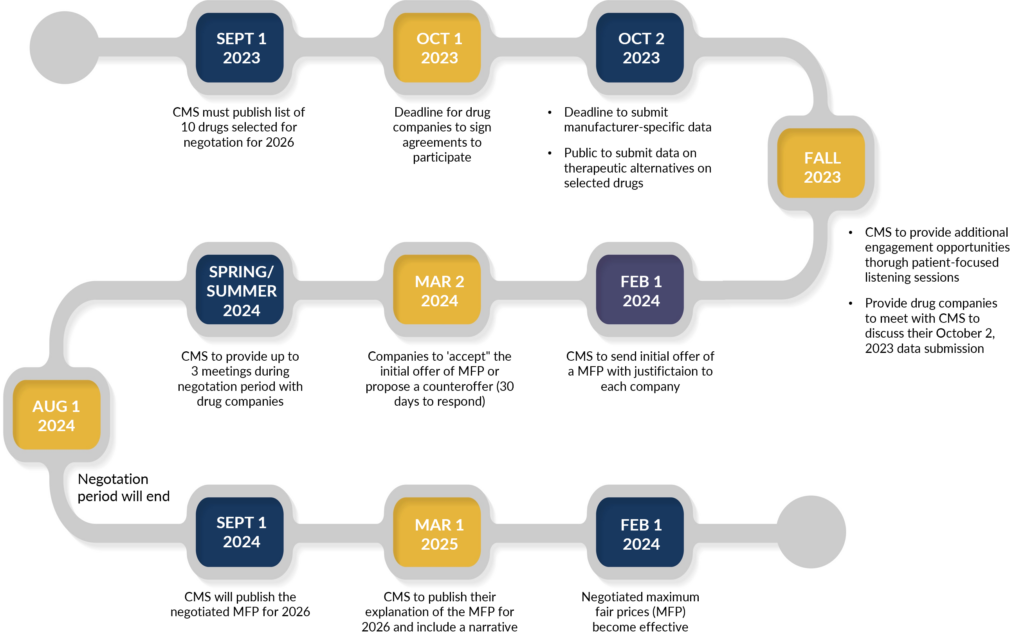
Another interesting piece of information: while the law provides for exceptions for small biotech, those that account for 1% or less of Part D or Part B spending and 80% or more of spending on that manufacturer’s drugs, only four drugs that applied have qualified for that exemption. Below is a timeline for price negotiations.
Price Negotiation Timeline & Key Dates
GET THE LATEST UPDATES ON IRA POLICIES AND GUIDANCE
Updates on new provisions, policies, guidance, and impacts of the IRA.
"*" indicates required fields

