How Pharma Manufacturers Can Navigate Additional Patient and HCP Support
1. Prepare in 2024 for Increased Financial Liabilities in 2025+
While the IRA imposes new financial burdens on manufacturers, including the need to perform rigorous financial forecasting to understand the long-term impacts, many need to prepare for the increased financial needs required. Here are the significant points to prepare for, with links to recently posted guidance:
- Training across their Sales teams, Field Reimbursement Managers (FRMs), Hub programs, and Specialty Pharmacies (SPs) to support questions from patients and HCPs
- Details on MPPP and if it will help a patient: here
- Explanation around Copay-smoothing to patients offered through multiple sources
- CMS MPPP Explanation & Video: here
- Checks and balances to ensure patient copays are calculated appropriate from month-to-month by their Part D plans
- CMS’ Monthly Bill Calculations: here
- Assurances that patient adherence is not impacted due to the delays related to enrolling into an MPPP/M3P program for new and/or existing Medicare beneficiaries
- Explanation on what happens after signing up: here
- Staff likely needed to monitor Part D plan coverage, re-enrollment and/or enrollment into a Part D plan, especially during reverification season and the new benefit year
2. Any Reactive Changes in Program Requirements Should Continue to Keep Patients First
Payers are likely to tighten restrictions and increase utilization management controls, potentially limiting access to specific therapies. Manufacturers need to be prepared for more aggressive cost-containment tactics such as step therapy (ST), quantity limits (QL), and stricter prior authorization criteria (PA). Most importantly, manufacturers should ensure shifts in coverage under a patient’s medical benefit vs. pharmacy benefit do not occur, given payers’ lack of awareness of implementing these changes in benefit design and coverage.
Manufacturers should also ensure that while Copay Smoothing is now available, requiring patients to enroll in an MPPP/M3P plan prior to being eligible for Patient Assistance Programs (PAP) may not be an immediate program change to make. Patients will need support, education, and appropriate timelines. Understand that all key stakeholders involved (i.e., SPs, Part D plans, etc.) will have long-hold times, may not be available to help, or may not even be prepared to answer questions. Hopefully, PAP Program eligibility criteria does not become overly restrictive, understanding the negative impact on patients and caregivers if implemented.
3. Data Management and Reporting Enhancements Needed
With quarterly reporting and new price caps, manufacturers will need to update CRM and data management systems for increased price reporting, program coverage, pricing transparency, and needs related to overall compliance. Such system updates require thoughtful planning with timelines in the 6-12 month range, not even including whether systems rely on data feeds vs. APIs, etc. This may increase administrative costs and necessitate significant updates, training, processes, and reporting needs.
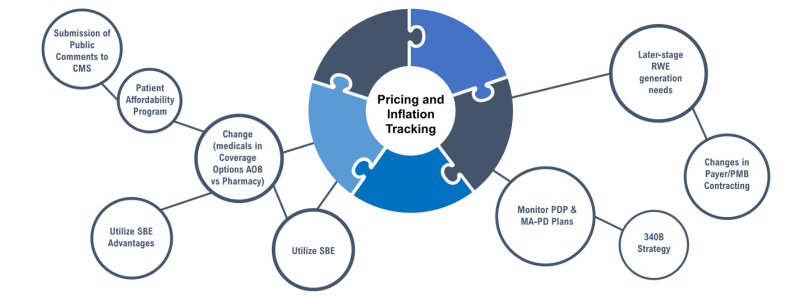
Recommendations for Pharmaceutical Manufacturers
Given the various impending changes beginning in 2025 and beyond, Manufacturers will want to ensure there are staff, systems, strategies, and budget to help patients and HCPs:
- Track product-specific changes in coverage, access, adherence, and financial responsibilities for patients, comparing those enrolled in MPPP vs those who are not
- Consider developing product-specific tools and resources (i.e., calculator) to help estimate cost-sharing and savings if enrolled into an MPPP
- CMS
- Connect patients with Foundations, if they are having trouble paying their Part D premiums and/or cost-share smoothed under an MPPP
- Train all stakeholders involved in helping patients access their medication on IRA, new 2025 Benefit Design, how to enroll into an MPPP, ready to follow-up on cost-share inaccuracies with billing/cost-share
Separately, from a financial risk/impact perspective, Manufacturers should evaluate:
- Medicare exposure and preparation for changes to existing contracting/rebating across all books of business.
- Conduct scenario planning exercises based on Medicare and competitor therapies to help indicate adjustments to long-term resource allocations anticipated/necessary/required
- Based on current payer policies and existing coverage limitations, consider additional investments in Real-world Evidence (RWE) generation, with positive/negative outcomes based on IRA
- Additional HEOR, long-term cost savings to systems, changes to outcomes and health systems, changes in payer behavior, etc.
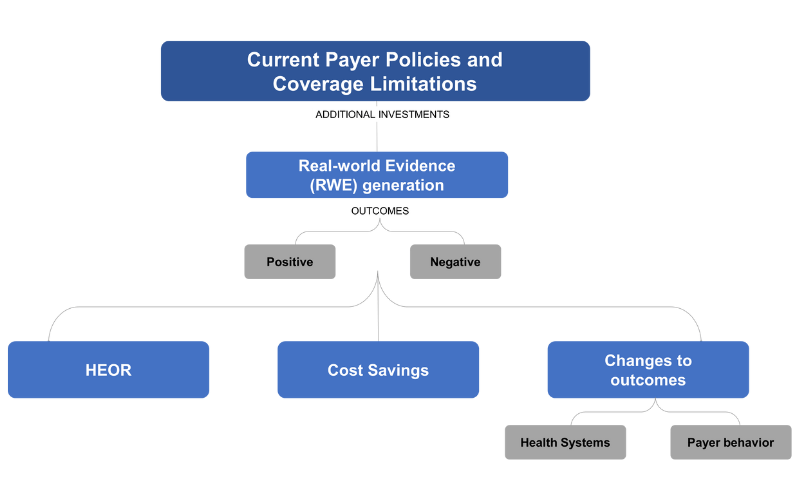
- Plan for additional publications around overall clinical benefit for patients based on claims data, chart reviews, AEs, outcomes, etc.
Given that the IRA will truncate a product’s time in the market at full price and potentially lead to additional types of negotiations outside of IRA provisions, manufacturers want to ensure products demonstrate long-term cost-effectiveness and value to payers, which will be key to sustaining pricing power in the commercial market.
GET THE LATEST UPDATES ON IRA POLICIES AND GUIDANCE
Updates on new provisions, policies, guidance, and impacts of the IRA.
"*" indicates required fields

